Minimally invasive surgery (MIS) has been at the center of several advances in medical diagnosis and treatments. From laparoscopic surgery, where surgical work is performed via small incisions, to endovascular surgery, where devices can image interior walls of arteries and treat problems using only one incision, these methods and devices play a pivotal role in modern healthcare as indispensable tools to improve and save lives. As the field continues to advance, optics and photonics product technologies will play an increasingly important role.
A key technology trend behind the proliferation of new, minimally invasive medical devices has been the miniaturization of high-resolution CMOS image sensors, enabling unique imaging modalities for enhanced visualization inside the human body. In addition, precision manufacturing and assembly of optics, mechanics, and light sources have made it possible to design and assemble medical devices less than 3 mm in size. Technology breakthroughs such as this are opening the door to more MIS applications, especially in the field of robot-assisted surgery. In parallel, the translation of existing 3D viewing technologies from the consumer electronics market is also a critical feature of new MIS products, especially in robotic surgery. The redesign and optimization of traditional 3D viewers for specific use cases in robotic-assisted surgery applications provide surgeons with new visualization and navigation capabilities, enable one to perform patient surgeries effectively and efficiently, ensuring better patient outcomes.
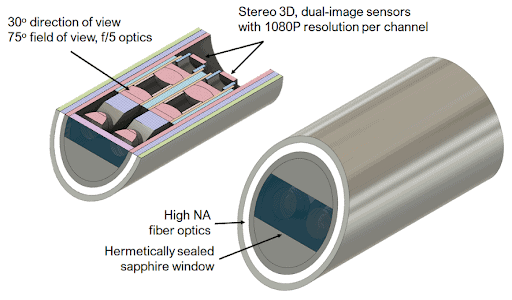
As new minimally invasive medical devices are developed based on optical visualization and sub-millimeter form factors, the requirements to (1) design and optimize the optical and mechanical package for each specific use case, (2) assemble and (3) accurately test and characterize the device to ensure the highest quality imaging performance requires the right product engineering approach, proven product development processes, and the right approach and ability to scale for manufacturing ramp-up to enable products to bring life-saving products to market faster.